Bioequivalence of topical generic products. Part 2. Paving the way to a tailored regulatory system - ScienceDirect
![PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f7e13872d81da37ef0bef45811f8303c900f3753/2-Figure1-1.png)
PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar

PDF) ASEAN GUIDELINES FOR THE CONDUCT OF BIOAVAILABILITY AND BIOEQUIVALENCE STUDIES | jessie wu - Academia.edu

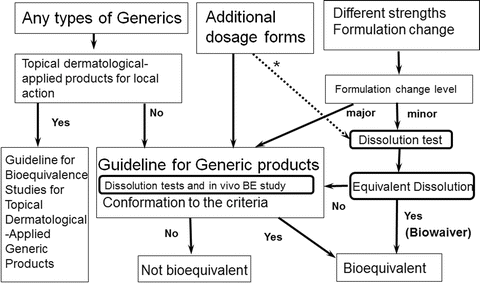
Current regulatory scenario and alternative surrogate methods to establish bioequivalence of topical generic products - ScienceDirect

GENERIC DRUGS: Guidelines for bioequivalence studies: Vishwakarma, Pushpendra Kumar: 9783639343779: Amazon.com: Books

Pharmacogenetic perspectives in improving pharmacokinetic profiles for efficient bioequivalence trials with highly variable drugs: a review | International Journal of Pharmacokinetics
Provisional Translation (as of October 2021)* Administrative Notice September 14, 2021 To: Pharmaceutical Affairs Section, Prefe
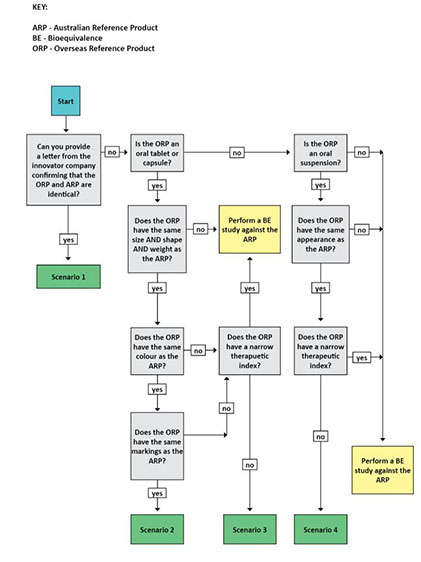
15.6 Choice of the reference product for bioequivalence of generic medicines | Therapeutic Goods Administration (TGA)

A pragmatic regulatory approach for complex generics through the U.S. FDA 505(j) or 505(b)(2) approval pathways - Klein - 2021 - Annals of the New York Academy of Sciences - Wiley Online Library